Usually, when things freeze – in other words turn from a liquid into a solid – they shrink or get smaller.
This is because, normally, if you make something hotter, it vibrates more. When it vibrates more, it tends to take up more space, so it tends to expand.
So, logically, if you cool something down, then the particles should move more slowly, collide and bounce off one another less hard and less frequently, and therefore, on average, spend more time closer together, making the material shrink.
Ice, on the other hand, is very unusual in that, as it gets colder, although the particles are certainly vibrating less for the reason explained above, it nonetheless expands or gets larger.
The reason for this is due to the strange shape of water molecules.
The oxygen atom is slightly negative, and the hydrogens are slightly positively charged, so water molecules tend to stick together forming what are called hydrogen bonds.
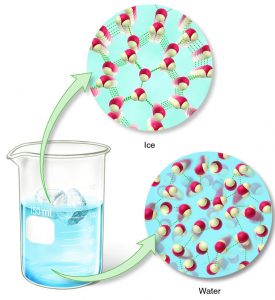
When water freezes, the molecules get themselves into the most stable configurations or positions that have the minimum amount of energy in the resulting ice crystal.
It so happens that the arrangement of water molecules that best satisfies this requirement is one that takes up even more space. And so ice expands when it freezes.






