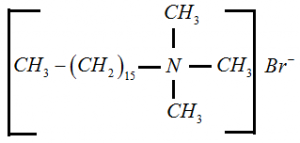If you are studying chemistry then you must have wondered about the importance of chemistry in everyday life. Chemistry is the branch of science which deals with the investigation of the properties and changes of matter. From the way how our body exchanges oxygen to how our universe was created, all have a side of chemistry associated with it.
Chemicals of Food in Everyday Life
In food materials following chemicals are widely used,
- Colouring agents
- Artificial preservatives
- Flow stabilizers
- Binding substance
- Artificial sweetness
- Antioxidants
- Minerals
- Vitamins
Except vitamins remaining substances do not have nutritional value.
Artificial Preservatives: These prevent spoilage of food by stopping the growth of microorganism. For example, Sodium benzoate, sodium meta bisulphate.
Artificial Sweetness: These do not impart any calories to the body. Since these substances are excreted through urine. For example,
- Aspartame: It is used in cool drinks and ice-creams.
- Alitame: It is 2000 times sweeter than sucrose.
Antioxidants: These prevent the spoilage of food by preventing the oxidation of food. For example,
- Butylated hydroxyl tolerance (BHT)
- Butylated hydroxyl anisole (BHA)
Dyes are coloured organic compounds that are used to impart colour to the various substrate, including paper, leather fur, hair drugs cosmetics. Dyes are classified into Natural dyes and Synthetic Dyes.
Chemistry of Cleansing Agents in Everyday Life
What are Soaps and Detergents?
Soaps are sodium or potassium salt of higher carboxylic acid such as stearic acid, Palmitic acid and oleic acid whereas detergents contain a long chain of alkyl groups. Detergents in comparison to soaps can also function in hard water.
Saponification: Alkaline hydrolysis of triesters of glycerol to form soap is known as saponification. Soaps do not function in hard water since they precipitate in it.
How do soaps work?
Soaps are generally sodium or potassium salts of long chain fatty acids. Soap molecules have a hydrophobic as well as hydrophilic part. While the hydrophilic part clings to the water when washing, the hydrophobic end clings to the dirt particles. Thus, when we pour away the water, the dirt particles wash away with the soap molecules.
Also Read: Cleansing Action of Soaps and Detergents
Types of Soaps
- Toilet Soaps: Potassium soaps are softer than sodium soaps.
- Floating Soaps: These can be prepared by beating soap bubbles.
- Transparent Soaps: This contains soap dissolved in excess of alcohol and it is evaporated.
- Medicated Soaps: These contain soaps by adding little amounts of Dettol, Savlon etc.
- Laundry Soaps mainly contains Sodium rosinate, borax.
Types of Detergents:
Anionic Detergent: In this, anion acts as detergents. For example, Sodium Lauryl Sulphate
Cationic Detergents: In this type, cation acts as a detergent. For example, Cetyl trimethyl ammonium bromide.
Non-Ionic Detergents: These are neutral. The whole molecule acts as a detergent. For example, Polyethylene glycol stearate.
Chemistry of Cosmetics in Everyday life
Cosmetics contains the following categories of chemicals.
- Emulsifier: These increase the stability of the emulsion. For example, Potassium cetyl sulfate.
- Preservatives: These are added to cosmetics to increase their shelf life. For example, benzyl alcohol, salicylic acid.
- Thickeners: These given an appealing consistency. For example, Cetyl alcohol, Stearic acid.
- Emollient: These soften the skin by preventing water loss. For example, Glycerine, zinc oxide.
- Glimmer and Shiners: For example, mica, bismuth oxychloride.
Other Examples of Chemistry in Everyday Life
Let us now discuss some common examples of chemistry in everyday life which most of us never knew about.
The Expiration Date on Bottled Drinking Water:
Have you ever wondered why there is an expiration date on a bottle of drinking water, after all, it is just water isn’t it? Well most of us haven’t even noticed that there is, in fact, an expiration date on that bottle. The idea behind instilling an expiration on bottled drinking water is to standardize the packaging quality of it.
What the actual expiration date signifies is if the expiration date is up, the taste of the water will be different as there is a chance of the chemicals in the packaging material ruining the quality of water.
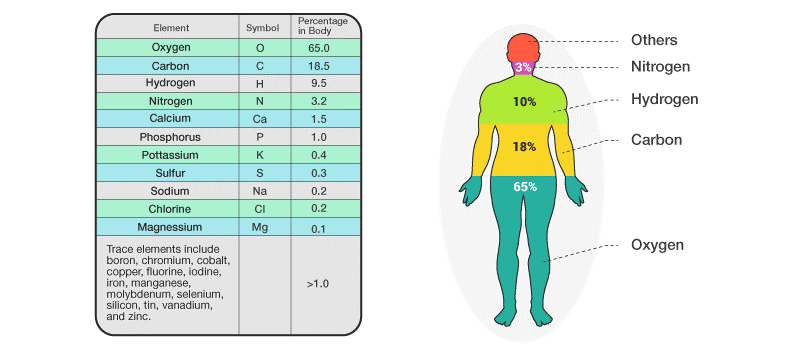
Elements in the Human Body:
We all know our body is about 60% water, but then what composes the rest of it? Carbon, Hydrogen, Nitrogen and Oxygen. These elements compose 96% of the human body. Whereas the rest 4% is composed of about 60 elements. Some of these elements include calcium, phosphorus, potassium, and sulphur.
Sunblock and Sunscreen:
There are two kinds of rays from the sun which are particularly bad for us, UV-A & UV-B. Sunscreen’s action is as the named suggest, it functions as a screen and offers protection from sunburns which is caused by UV-B. Whereas sunblock has more of reflective nature and blocks both UV-A & UV-B radiations.
© Chemistry In Everyday Life – Importance, Examples, Uses (byjus.com)