Cato Maximilian Guldberg was born on this day in 1836. Norwegian chemist who, with his brother-in-law Peter Waage, formulated the law of mass action (1864), which details the effects of concentration, mass, and temperature on chemical reaction rates. The law states that the rate of a chemical change depends on the concentrations of the reactants. Thus for a reaction: A + B -> C the rate of reaction is proportional to [A][B], where [A] and [B] are concentrations. In 1870, Guldberg investigated the way in which the freezing point and vapor pressure of a pure liquid are lowered by a dissolved component. In 1890, he formulated Guldberg’s law which relates boiling point and critical temperature.
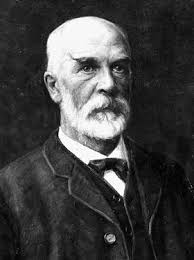
August 11th
200800login-checkAugust 11th





