Thin-layer chromatography (TLC) is a chromatographic technique used to separate non-volatile mixtures. Thin-layer chromatography is performed on a sheet of an inert substrate such as glass, plastic, or aluminium foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminium oxide (alumina), or cellulose. This layer of adsorbent is known as the stationary phase.
TLC is an analytical tool widely used because of its simplicity, relative low cost, high sensitivity, and speed of separation. TLC functions on the same principle as all chromatography: a compound will have different affinities for the mobile and stationary phases, and this affects the speed at which it migrates. The goal of TLC is to obtain well defined, well separated spots.
Technique
The process is similar to paper chromatography with the advantage of faster runs, better separations, and the choice between different stationary phases. Because of its simplicity and speed, TLC is often used for monitoring chemical reactions and for the qualitative analysis of reaction products. Plates can be labeled before or after the chromatography process using a pencil or other implement that will not interfere or react with the process.
To run a thin layer chromatography plate, the following procedure is carried out:
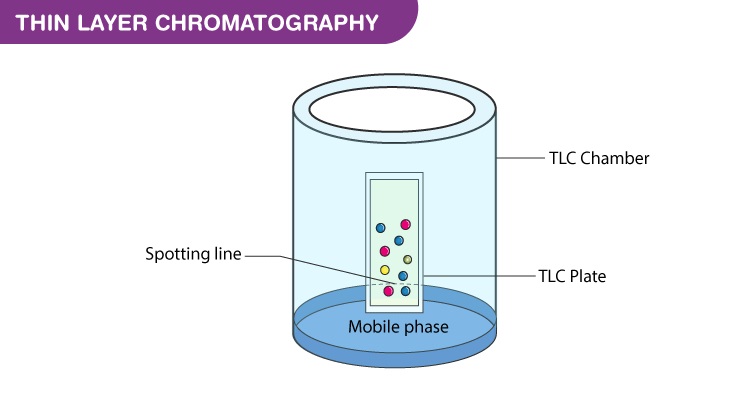
- Using a capillary tube, a small spot of solution containing the sample is applied to a plate, about 1.5 centimeters from the bottom edge. The solvent is allowed to completely evaporate off to prevent it from interfering with sample’s interactions with the mobile phase in the next step. If a non-volatile solvent was used to apply the sample, the plate needs to be dried in a vacuum chamber. This step is often repeated to ensure there is enough analyte at the starting spot on the plate to obtain a visible result. Different samples can be placed in a row of spots the same distance from the bottom edge, each of which will move in its own adjacent lane from its own starting point.
- A small amount of an appropriate solvent (eluent) is poured into a glass beaker or any other suitable transparent container (separation chamber) to a depth of less than 1 centimeter. A strip of filter paper (aka “wick”) is put into the chamber so that its bottom touches the solvent and the paper lies on the chamber wall and reaches almost to the top of the container. The container is closed with a cover glass or any other lid and is left for a few minutes to let the solvent vapors ascend the filter paper and saturate the air in the chamber. (Failure to saturate the chamber will result in poor separation and non-reproducible results.)
- The TLC plate is then placed in the chamber so that the spot(s) of the sample do not touch the surface of the eluent in the chamber, and the lid is closed. The solvent moves up the plate by capillary action, meets the sample mixture and carries it up the plate (elutes the sample). The plate should be removed from the chamber before the solvent front reaches the top of the stationary phase (continuation of the elution will give a misleading result) and dried.
- Without delay, the solvent front, the furthest extent of solvent up the plate, is marked.
- The plate is visualized. As some plates are pre-coated with a phosphor such as zinc sulfide, allowing many compounds to be visualized by using ultraviolet light; dark spots appear where the compounds block the UV light from striking the plate. Alternatively, plates can be sprayed or immersed in chemicals after elution. Various visualising agents react with the spots to produce visible results.
References
- Touchstone, Joseph C. Practice of thin layer chromatography. 2nd ed. New York: Wiley, 1983.Print.
-
Geiss, Friedrich. Fundamentals of thin layer chromatography planar chromatography. Heidelberg: A. Hüthig, 1987. Print.
-
Touchstone, Joseph C. Practice of thin layer chromatography. 3rd ed. New York: Wiley, 1992. Print.