Trace amounts of olefins and diolefins found in gasoline are prone to reaction with oxygen dissolved in the gasoline. This process is referred to as autoxidation and involves a radical chain reaction that can incorporate oxygen
into the olefin and also can promote a molecular size increase via polymerization reactions. The end result of this complex process is the formation of deposits and gums that can block fuel filters and interfere with the metering of fuel and air in the carburetor. This can result in adverse engine performance. Additives are frequently added to gasoline to address oxidative stability and other issues; they include antioxidants, metal deactivators, and detergents.
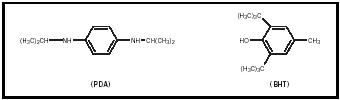
Antioxidants are additives that minimize autoxidation reactions. They function as hydrogen atom donors that stop the chain oxidation process of the olefins. The two different types of antioxidants used in gasoline are phenylenediamines (PDA) and hindered phenols (such as BHT).
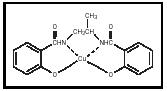
Trace levels of soluble metal compounds, particularly copper, catalyze the oxidative degradation of gasoline by promoting the formation of gums and deposits. Metal deactivators overcome this problem by chelating the metal and rendering it inactive. The most widely used metal deactivator is N, N′-disalicylidene-1,2-propanediamine, the copper complex of which is shown in Figure 3.
Detergents minimize fuel system deposits at low concentrations, and at high concentrations can remove deposits that have already formed. Detergents are molecules that have a highly polar end group and a nonpolar hydrocarbon tail. A conventional amino amide type detergent is shown in Figure 4.
Presumably, the polar groups in the detergent attach themselves to metal surfaces and to polar deposits on these surfaces. The nonpolar tails of these molecules “stick out” into the fuel in such a way that a monomolecular film is formed on the metal surface, preventing deposition and particle aggregation. This process is also believed to solubilize any deposits already on the metal surface. The detergent monolayer is also believed to prevent the buildup of ice on carburetor surfaces during winter. Thus, detergents can also function as anti-icing additives.